Abstract
Though remarkable progress has been made in the field of multiple myeloma (MM), with proteasome inhibitors, immunomodulators and monoclonal antibodies, the disease is still currently incurable. Outcomes are dismal when patients become refractory to bortezomib, lenalidomide, pomalidomide and daratumumab (quadri-refractory). Several new treatment approaches, including enhanced monoclonal antibodies, antibody-drug conjugates (ADC), bispecific T-cell engagers (BiTE) and chimeric antigen-T-cell therapy (CAR-T) are under development. Antibody-based therapeutics targeting CD38 and SLAMF7 and a cellular therapy targeting BCMA have now been FDA-approved. However, most patients still relapse even after BCMA CAR-T infusion. New designs such as HLA-independent T cell receptors showed a high efficacy in the context of low antigen density, so new targets are now accessible for the treatment of tumors. Our objective was to identify novel targets in relapsed or efractory MM patients and to allow the development of an original treatment.
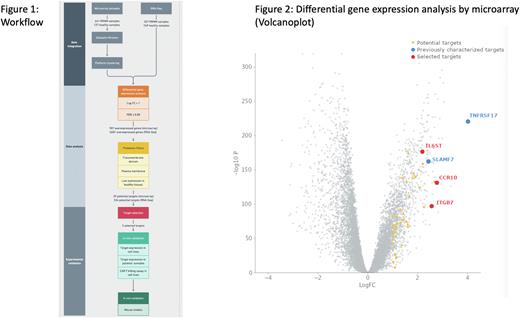
To do so, we integrated transcriptomic and proteomic data from malignant plasma cells and healthy donor samples, developed an analysis pipeline and further validated selected targets. We created cohorts comprising both RRMM patients and healthy donor samples using 11 publicly available microarray datasets and 2 RNA sequencing datasets (MMRF-CoMMpass and GTEx), including a total of 830 and 1018 samples respectively (Fig. 1). We included samples taken both at diagnosis and at event to explore the stability of the expression of potential targets. We then performed a differential gene expression analysis followed by proteomic filtering to select solely surface antigens displaying a transmembrane domain and a low expression in healthy tissues in both cohorts. We subsequently prioritized potential targets based on multiple criteria, including their presence in multiple analyses (e.g., RNA-Seq, microarray, at diagnosis, at event), as well as the availability of additional supporting evidence (e.g., tissue-level and subcellular-level expression across multiple proteomic databases).
Using this approach, we not only re-discovered well characterized multiple myeloma targets, such as B cell maturation antigen (BCMA) or SLAM Family Member 7 (SLAMF7), but we also identified promising targets, such as C-C Motif Chemokine Receptor 10 (CCR10), Integrin Subunit Beta 7 (ITGB7) and Interleukin 6 Cytokine Family Signal Transducer (IL6ST) (Fig. 2).
We further conducted experimental validation of the protein expression of these promising targets using flow cytometry in MM cell lines (MM1S, OPM2). We also found that high expression of ITGB7 is higher in MM cells lines compared to other tumor cell lines (according to CCLE database), predictive of worse progression free survival in myeloma patients, and maintained at relapse. Finally, given the high traction of cell therapy approaches in innovative myeloma traitement strategies, we sought to establish a proof-of-concept chimeric antigen receptor (CAR) T-cell versus CCR10 (4-1BB-based, 2nd generation CAR and lentivirally transduced primary human CD8+ T cells). Encouragingly, we observed that CCL27-based CAR-Ts with CCR10 knockout exhibited in vitro killing activity against MM.1S myeloma cells.
In conclusion, our translational bioinformatics approach yielded novel antigen targets amenable for Chimeric Antigen Receptor-T (CAR-T) cell and HLA-independent T cell receptors (HIT receptors) approaches, potentially promising for the treatment of RRMM multiple myeloma patients.
Disclosures
Weill:Epigene Labs: Current Employment, Current equity holder in private company. Fox:Epigene Labs: Current Employment, Current equity holder in private company. Meunier:Epigene Labs: Current Employment, Current equity holder in private company. Appé:Epigene Labs: Current Employment, Current equity holder in private company. Behdenna:Epigene Labs: Current Employment, Current equity holder in private company. Wiita:Genentech: Research Funding. Nordor:Epigene Labs: Current equity holder in private company, Other: Compensated co-founder. Eyquem:Mnemo Therapeutics: Current equity holder in private company, Other: Compensated co-founder; Cytovia Therapeutics: Consultancy, Current equity holder in private company, Research Funding; Casdin Capital: Consultancy; Takeda: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal